Water is Weird
- Zahra
- Mar 1, 2023
- 4 min read
Hello again! Today we will be discussing how odd water really is. I mean, we see it all the time, and it is one of the biggest reasons humans are around, but really looking into water just reveals how odd of a substance it truly is.
Water is one of the most common and vital substances on our planet, it being the fundamental solvent needed for life to exist as we know it. It is required for all kinds of processes in living beings, from the little things like cellular processes to ensuring we digest our food properly and has many strange qualities that enable it to sustain life.
However, water did not even originate from our own planet. The oceans we know of did not form until millions of years after the formation of the plant, and scientists currently believe that this only occurred once asteroids carrying water collided into the planet, providing the planet with the liquid it needed to sustain life. However, there are other theories that are also being investigated, which sounds a little odd, since you would think we would know where water came from by now, but hey.
What we do know, however, is that we can make it in a laboratory setting, but it is both impractical and strenuous to do, especially since it requires a lot of energy to combine the hydrogen and helium molecules, which is how we know that water is not simply made. From this, scientists believe that the water that is on our planet has been recycled repeatedly, to be found in living beings, rocks, in our atmosphere; almost everywhere on our planet. It is a very good solvent, meaning it is very good at breaking down substances (far better than any other common liquid), but also means that it is very difficult to find in its pure form naturally.
One of the most interesting things to note, however, is that water behaves unlike any other we know of when in the temperature range that our planet naturally exists in, only acting like other liquids at extreme temperatures, which means we can experience the strange behaviours of water in our day-to-day lives and learn just how strange water is.
For example, water is the only known substance to expand when freezing. Water is most dense at 4°C, before the molecules form tetrahedral arrangements and create more free space than the liquid equivalent, as can be seen below:
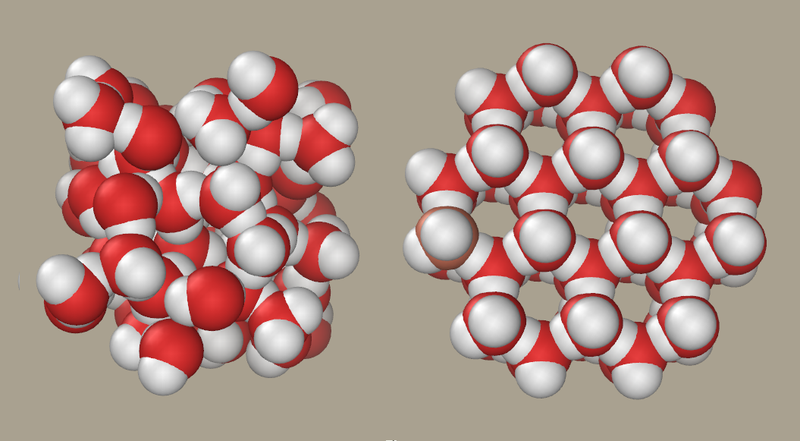
Liquid water and ice. Credit: P99am
The arrangement of these molecules is the reason for floating ice: the structure of liquid water is denser than that of ice – unlike other chemicals which become denser when freezing – which means that the less dense ice will float to the top of the water.
This plays a huge role in the biochemistry of our planet, as large bodies of water would freeze over if ice did not float to the surface, and instead the layer of ice on the water allows heat to be trapped within the water and prevent it from freezing over. Without this, life as we know it would not have been able to develop, since aquatic life would have been frozen through a very long time ago. In having a place of safety from the bitter cold, organisms had a way of surviving and further developing, and far more fragile life-forms were then able to further grow and develop into those we know of today.
Beyond this, however, scientists are not quite sure how this process of freezing happens and are still trying to investigate just what happens on a molecular level.
Another confusing phenomenon to add to this mess is the Mpemba effect, where a student from Tanzania discovered that hot water freezes much faster than cold water. This doesn’t quite make intuitive sense, and yet, it has been scientifically proven to only occur under certain conditions, those of which scientists are still trying to figure out.
The way this experiment is carried out is by heating up some water, then cooling it down to the same temperature as another sample of water. When you start freezing these water samples, you would find that the water that was initially heated up will freeze faster than the second water sample. Of course, there are many factors that scientists believe play into this effect, although it is a strange phenomenon that you could easily experiment with on your own, although I would be careful any hot water if you plan to do this yourself.
The next strange thing is that water shouldn’t even be a liquid to start with – other compounds such as carbon dioxide or ammonia consist of heavier atoms, and yet are gases at room temperature, while somehow water has a freezing point of 0°C (carbon dioxide has a freezing point of -78°C for comparison). The boiling and freezing point of water also makes it the only substance on earth that can be naturally found in all three states, due to the strangely large disparity between the two values. Again, this has a stark difference to other molecules of similar size and structure, and yet water being a liquid has been and still is incredibly vital for life.
Water also has an incredible amount of surface tension, due to the strong attraction between oppositely charged particles. This is because these hydrogen bonds that are formed are far stronger than other seemingly similar compounds, and end up creating a uniquely cohesive liquid, that has a far greater surface tension and makes it a very good solvent. This is why it is almost impossible to find water in its pure form, since it will pretty much only be found as a part of a solution with other molecules.

This diagram shows just how hydrogen bonds work
This high surface tension makes water almost sticky, since these hydrogen bonds take far more energy to break than those usually found between molecules. This is how you can have strange situations where you can have more water in a cup than the cup can hold; the hydrogen bonds hold the water molecules together incredibly well. Because of this phenomenon, we can also see how water is the most incompressible liquid, with even oceans hardly experiencing any compression, even if several kilometres deep.
Overall, water is one of the strangest substances we have on our planet, and there are so many strange things about water that we hardly know about, despite it being one of the biggest factors that play into life on our planet.
Comments